Carbon and its Compounds || Class 10 || Science || CBSE Notes
Dear Students, Today we are going to share the Notes of Carbon and its Compound. These notes are prepared by the experienced teachers. These notes are free to all the students. These notes has been prepared according to the CCE pattern of school education based on NCERT Syllabus prescribed by the Central Board of School Education for Class X. All the important topics are covered in these notes.
Some Important Topics
✴ Functional Group
❇Halo Group
❇Alcohol Group
❇Aldehyde Group
❇Ketone Group
❇Carboxylic Acid Group
✴Coal and Petroleum
❇Coal formation
❇Petroleum formation
❇Substances burn with a flame or
without flame
✴Ethanol (or Ethyl Alcohol)
❇ Preparation of Ethanol
❇ Physical Property of Ethanol
❇ Chemical Property of Ethanol
✴ Combustion
✴ Oxidation
✴ Reaction with sodium metal
❇ Dehydration of Ethanol
❇ Uses of Ethanol
✴ Harmful Effect of Drinking Alcohol
❇ Denatured Alcohol
❇ Test for Alcohol
✴ Sodium Metal Test
✴ Sodium Metal Test
✴ Easter Test
✴ Ethanoic Acid or Acetic Acid
❇ Physical Property of Ethanoic Acid
❇ Chemical Property of Ethanoic Acid
✴ Action on Litmus
✴ Dilute Ethanoic
✴ Reaction with Sodium H -Carbonate
✴ Reaction of Ethanoic acid with
Alcohols
❇ Uses of Ethanoic Acid
❇ Test for Ethanoic Acid
✴ Shops
❇ Manufacture of Soap
❇ Structure of Soap
❇ Cleansing Action of Soap
❇ Limitation of Soap
✴ Detergents
Functional Group
An atom or a group of
atoms which makes a carbon compound (or Organic Compound) reactive and decides
its properties is called a functional
group.
The alcohol group –
OH, present in ethanol C2H5OH, is an example of
functional group. Some of the important functional groups are Halo group (or
Halogeno group). Alcohol group, Aldehyde group, Ketone group, Carboxylic Acid
group, Alkene group and Alkyne group. These are discussed below:
1. Halo Group :ªX (X can ve Cl, Br or I)
The halo group can be
chloro- Cl, bromo – Br, or iodo- I , depending upon whether a chlorine, bromine
or iodine atom is linked to a carbon atom of the organic compound.
Chloro group is
present in Chloromethane CH3 ― Cl,
Bromo group is present
in bromomethane CH3 ― Br
And Iodo group is
present in Iodomethane CH3 ― I
The haloalkanes can be
written as (R ― X ) where R is an alkyl group and X is the halogen atom.
(2) Alcohol Group:-
OH
The alcohol group is made up of one oxygen atom and one
hydrogen atom joined together. The alcohol group is also known as alcoholic group
or hydroxyl group. The compound containing alcohol group are known as alcohols.
Example of compound containing alcohols group are : methanol CH3OH,
ethanol C2H5OH.
The general formula can be written as R―OH where R is alkyl
group and OH is the alcohol group.
Aldehyde Group:-
The aldehyde group consists of one carbon atom, one hydrogen atom and one oxygen atom joined together.
The aldehyde group consists of one carbon atom, one hydrogen atom and one oxygen atom joined together.
or we can say that these are the organic compound which
contain ―CHO group (i.e. aldeyde group as a functional group) attached to a
c―atom of an alkane molecule.
Ketone Group :
These are the organic compounds which contains ―co― group (ketone
gp) as a functional group attached to a c―atom of an alkane molecule.
Some example are :- CH3COCH3
(Propanone), CH3COCH2CH3 (Butanone)
A Ketone group is always between two alkyl group. It can
never be at the end of a carbon chain because it has two valency which have to
be satisfied by two alkyl groups.
Carboxylic Acid Group:
These are the organic compound which contains ― COOH group as a functional group attached
to a c―atom group as a functional group attached to a c―atom of alkane.
For example:- methanoic acid H―COOH and ethanoic acid CH3―COOH.
For example:- methanoic acid H―COOH and ethanoic acid CH3―COOH.
Coal and Petroleum
When a fuel is burned, the energy is released mainly as heat
and some light, Most of the fuel which we can used today are obtained from coal,
petroleum and natural gas.
Coal is complex mixture of compounds of carbon, hydrogen and
oxygen and some free carbon. Small amounts of Nitrogen and sulphur compounds are
also present in coal.
How Coal was formed
Coal was formed by the decomposition of large plants and trees
buried under the trees million years ago. It is believed that millions of year
ago, due to earthquakes and volcanoes etc, the forests were buried under the
surface of the earth and got covered with sand, clay and water. Due to high
temperature and high pressure inside the earth, and in the absence of air would
was converted into coal.
How petroleum was
formed :- Petroleum
oil were formed by the decomposition of remains of extremely small plants and
animals buried under the sea millions of
years ago.
Why do substances burn with a flame
or without flame
(1) When the oxygen supply is sufficient then the fuel burn
completely producing a blue flame. In a gas stove or cooking gas there is sufficient
oxygen so produce much heat but less light. Therefore, it is said to be in non-luminous
(or non light giving) flame.
(2) When the oxygen supply is insufficient then the fuels
burns incompletely producing mainly a yellow flame. The yellow flame producing
light so it is said to be luminous flame.
Ethanol (or Ethyl
Alcohol)
Preparation of Ethanol:- Ethanol is commercially prepared by
the fermentation of sugar present in the molasses by the action of enzyme
called invertase and zymase secreated by yeast at a control temperature of 200
C ― 300 C
The vessel used for fermentation is design in such a way that
it allows CO2 to escape out of it, but does not allow the fresh air to
enter into the vessel as it can oxidised Ethanol
to Ethanoic acid.
Physical Property of Ethanol:-
(a) It is a colorless
liquid with pleasent smell.
(b) Its boiling point
is 780 C.
(c) It is lighter than
water.
(d) It is soluble in
water.
(e) It remains as
liquid even in winter.
(f) It is neutral.
(g) It is covalent
compound.
(h) It is a poor
conductor of electricity.
(i) It catches fire
easily.
Chemical Property of
Ethanol:
(a) Combustion:- Ethanol
burns in air to produce CO2 water vapours and lot of heat as
(b) Oxidation:-
When ethanol is heated with alkaline potassium permanganate solution (or
acidified potassium dichromate) it gets oxidized to ethanoic acid.
(c) Reaction with
sodium metal :- Ethanol react with sodium to form sodium ethoxide and
hydrogen gas.
Reaction with Carboxylic acids or with ethanoic acid :-
Ethanol reacts with ethanoic acid in the presence of concentrated H2SO4
to produce a sweet smelling ester as ethyl ethanoate:
Dehydration of Ethanol:- When ethanol is heated with excess
of conc Sulphuric acid at 1700 C , it get dehydrated to form ethane.
Uses of Ethanol
(1) Ethanol is used in the manufacture of paints, varnishes,
liquiors, medicines, perfumes, dyes, soap
and synthetic rubber.
(2) Ethanol alcohol is used as a solvent. Many organic
compounds which are insoluble in water are insoluble in water are soluble in
ethyl alcohol.
(3) Being a good solvent, it is used in medicine such as as
tincture iodine, cough syrups and many tonics.
(4) Ethyl alcohol is used as a fuel in cars along with petrol.
it is used as a fuel in spirit lamps.
(5) It is used in alcoholic drinks like whisky, wine beer and
other liquors.
(6) It is used as an antiscptic to sterilize wounds and
syringes in hospital and dispensaries.
Harmful Effect of
Drinking Alcohol
(1) Alcohol slows down the activity of nervous system and the
brain due to be the judgment of a person is impaired and his reaction become
slow. So a person driving a car under the influence of alcohol cannot judge a
situation properly and act quickly in case of an emergency.
(2) Alcohol drinking inhabitation (mental restrain) due to
which a drunken man become quarrelsome. This leads to quarrels and fights which
increase violence and crime in society.
(3) Drinking alcohol heavily land of straggered movement,
slurred speech, blurred vision and vomiting.
(4) Heavy drinking of alcohol over a long time may damage the
stomach, liver, heart and even brain.
(5) Heavy and continuous drinking of alcohol makes the person
bankrupt.
(6) The drinking of adulterated alcohol contain methyl
alcohol (methanol) cause serve poisoning leading to brightness and even death.
Denatured Alcohol :- To prevent the misuse of
industrial alcohol for drinking purposes (or black marketing) ethyl alcohol meant
for industries is denatured by adding some amount of poisonous substance like methanol
, pyridine or copper sulphate, etc. So denatured alcohol is ethyl alcohol which
has been made unfit for drinking purpose by adding small amount of poisonous
substance like methanol.
Test for Alcohol
(1) Sodium Metal
Test:- If on adding sodium metal to the organic liquid, the bubble of
hydrogen gas are produce then it indicate that the given organic liquid is
ethanol.
(2) Easter Test:-
If on warming solution with some glacial ethanoic acid and a few drop of cons
sulphuric acid a sweet smell produced that is ester produce, it indicate that
solution is ethanol.
Ethanoic Acid or Acetic
Acid
Ethanoic acid can be prepared by the oxidation of Ethanol
which can be done either by alkaline KMnO4 or acidic K2Cr2O7
Physical Property of Ethanoic Acid:
(a) It is a colorless liquid having a sour taste
and a pungent smell.
(b) Its boiling point is 1180 C.
(c) Pure ethanoic acid
is called glacial ethanoic acid (or glacial acetic acid).
(d) It miscible with
water in all propotions.
(e) It is a good
conductor of electricity.
Chemical Property of Ethanoic Acid
(a) Action on Litmus:- Ethanoic acid turn blue Litmus to Red
as it produce H+ (aqions)
in the solution.
(b) Dilute Ethanoic acid turn indicator paper to Orange showing
that pH is about 4 which tell us that
it is a weak acid.
(c) Reaction with
Sodium H -Carbonate
Ethanoic acid react with sodium hydrogen-carbonate to evolve
brisk effervescence of carbon dioxide gas:
This reaction is used as a test of ethanoic acid.
Reaction of Ethanoic
acid with Alcohols:-
It reacts with alcohols in the presence of little of
concentrated Sulphuric acid to form Easter.
Example: Easter are usually volatile liquids having sweet
smell or pleasant smell.
Uses of Ethanoic Acid:
(1) Dilute ethanoic
acid in the form of vinegar is used as a food preservative in the preparation
of pickles and sauces.
(2) Ethanoic acid is
used in the manufacture of acetone as ester used in perfumes.
(3) It is used for
making cellulose acetate which is an important artificial fibre.
(4) It is used in
preparation of dyes, plastics and pharmaceuticals.
(5) It is also used to
coagulate rubber from latex.
Test for Ethanoic Acid:
If a pinch of sodium bicarbonate is added to ethanoic acid,
the evolution of CO2 gas with brisk effervescence show that the
given organic compound is carboxylic acid.
Shops
A soap is the sodium or potassium salt of long chain
carboxylic acid (fatty acid) which has cleansing property in water. Example of
soaps are:- Sodium Stearate and Sodium palmitate.
Manufacture of Soap:- Soap is made by heating animal fat
or vegetable oil with concentrated sodium hydroxide solution (caustic soda
solution)
General reaction:
General reaction:
The process of making shop by hydrolysis of fats and oil with
alkalis is called saponification. The saponification gets completed soap is obtained
in the form of solution. Common salt is then added to precipitate out all the
soap from the aqueous solution. It decrease the solubility of due to which all
the soap separate out from the solution in the form of liquid.
Structure of Soap:
A soap molecules is made up of two parts: a long hydrocarbon
part and a short ionic part containing ― Coo‾
Na+ group.
When Soap is dissolved in water it forms a colloidal
suspension in water in which the soap molecules cluster together to form
spherical misclles as in figure.
Micelle formation takes place when soap is added to water
because the hydrocarbon chain of soap molecules (water repelling) which are insoluble
in water but the ionic end of soap molecules are hydrophilic (water attracting)
and hence soluble in water.
When the dirt cloth is agitated in soap solution, the oily
and greasy particle present on its surface and entrapped by soap micelles get
dispersed in water due to which the soap water become dirty but the cloth get
clean.
Limitation of Soap
Following are the limitation of soap:-
(1) Soap is not suitable for washing clothes with hard water,
it is because soap reacts with calcium and magnesium salts to form insoluble
precipitate called scum which cloth a dirty appearance.
Detergents:
Detergents are also called soap less soaps because though
they act like a soap in having cleansing properties they do not contain the
usual soaps like sodium stearate etc.
Detergent are better cleansing agents than the soap because
they can be used for washing even in hard water.
A detergent in the sodium salt of long chain benzene sulphonic
acid (or the sodium salt of long chain alkyl hydrogen sulphate) which as
cleansing properties in water.
Soap
|
Detergent
|
|
1
|
It is the sodium salt of long chain carboxylic or
fatty acid which has cleansing action in water.
|
It is the sodium salt of long chain.
(a) Benzene Sulphonic acid.
(b) alkyl hydrogen sulphate which has cleansing
action in water.
|
2
|
Example: Sodium Stearete C17H35‾
Coo―Na+
|
Example: Sodium-n-dedecyl benzene sulphonate.
|
3
|
They are obtained from animal fats and vegetable
oils.
|
They are obtained from petroleum products.
|
4
|
They are biodegradable and hence does not cause
pollution.
|
They are biodegradable and hence cause pollution
|
5
|
They have relatively weaker cleansing action.
|
They have a stronger cleansing action.
|
6
|
They do not work well in hard water.
|
They work well in hard water.
|
7
|
They form scum in hard water
|
They do not form scum in hard water.
|
8
|
The short ionic part is Coo―Na+
(hydrophilic).
|
The short ionic part is So‾3 Na+ or SO4 Na+.
|














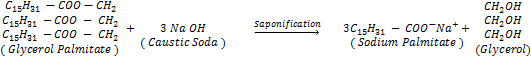



Comments
Post a Comment